Secure & Compliant Infrastructure On-demand for Pharma & Life Sciences in the Cloud
Sudish Mogli - Technology Advisor,
Gautham Gurumurthy - Principal Technical Program Manager
Jan 20, 2021
Secure and compliant Infrastructure is one of the fundamental requirements for Pharma and life Sciences companies in the cloud. These industries require scalable compute and storage capabilities to run advanced algorithms and processes to tabulate drug and reagent performance, to increase the overall drug efficacy. The Infrastructure must be GxP and HITRUST certified to ensure developed drugs can pass regulatory approvals. This is required for the delivery of highly personalized healthcare, improving the quality of evidence collected from Real-World Data and enhancing the Pharmacovigilance (Drug Safety) aspect of drug development and research.
Cloud Infrastructure changed the industry paradigm by providing infrastructure on-demand. It afforded the opportunity for industries to take advantage of Infrastructure as Code (IaC) to rapidly and consistently spin up infrastructure. However, this advancement is still new, without deep expertise in infrastructure code development, public cloud infrastructure, security and compliance, most public cloud consumers are still struggling to develop and deploy code artifacts that strike the appropriate balance between the critical tiers of Application Hosting & Data processing with Security, Compliance & Performance. This makes public cloud adoption much harder for any industry especially regulated industries such as the healthcare and pharmaceutical industries.
The healthcare and life science industry globally is embracing digitization considering the rapidly increasing healthcare expenditure (forecast to touch $10 trillion by 2022 as per the 2019 Global Health Care Outlook by Deloitte). This drive for digitization is pushing Healthcare & Life Sciences industries to explore and evaluate public cloud in a more critical light. Though IaC has its challenges, it also comes with capabilities which enable paradigms such as continuous compliance and intelligent infrastructure. We believe that the following graph demonstrated our vision as to how IaC will evolve in the next few years, and it’s very critical for organizations to stay in line with this curve.
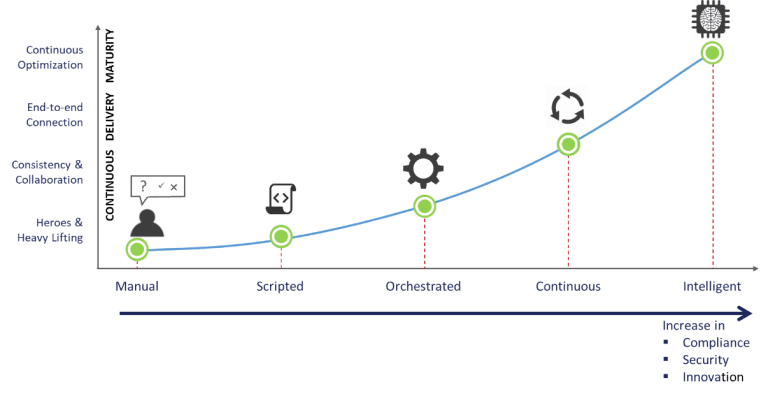
Here is where we come in. The HCTI’s HITRUST certified CloudEz comes with Cloud Infrastructure Accelerator Toolkit & APIs to provide an end-to-end solution to Develop and Operate your cloud infrastructure. The Toolkit and APIs provide several ready to deploy infrastructure templates for public cloud which have security, compliance and performance controls built in. The IaC templates account for automatic GxP records like IQR, OQR and PQRs as well as the security controls to meet HITRUST certification. These IaC templates are API enabled, to orchestrate end user services. End User Services that remain continuously compliant and generate a compliance trail for an always audit ready cloud platform. The End User Services can be leveraged to rapidly build a repeatable enterprise-ready cloud ecosystem in the public cloud. This allows our customers to focus on developing and deploying their applications on a baseline secure and compliant environment.
HCTI’s vast repository of deployable infrastructure code artifacts which have prebaked security, GxP, HITRUST and performance controls allow Life Science and Healthcare Industries to focus on Drug research, Trials and Manufacture instead of infrastructure. Customers rely on HCTI for our deep knowledge and HITRUST certified process and procedures built over the years working with Pharma, Life Sciences and Healthcare companies. With over 10 years of Public Cloud experience, combined with our expertise with the development, deployment, security and compliance requirements of Pharma and Healthcare verticals, we have been able to deploy and manage highly sensitive applications on the Public Cloud for our customers.
For queries or any help on how to choose the right public cloud framework with continuous security and compliance for your pharma/ healthcare/ life sciences organization, please contact us at info@healthcaretriangle.com


